Air Fuel Ratios and Stoichiometry
 Burning fuels is a chemical reaction,
and has to abide by a number of rules. Petrol is a Hydrocarbon, which is
made of Hydrogen and Carbon. Other additives in the petrol also add more elements, but
for the purpose of this article, we'll just look at the hydrocarbons.
Air is a mixture of gasses, mostly Nitrogen which is quite unreactive under normal
conditions, and there is also about 21% Oxygen. The Oxygen reacts with the hydrocarbons when burnt
to produce Carbon Monoxide (CO), Carbon Dioxide (CO2) and Water (H2O).
Under the temperatures and pressures involved, Nitrogen also forms some oxides, collectively
referred to as NOX.
Burning fuels is a chemical reaction,
and has to abide by a number of rules. Petrol is a Hydrocarbon, which is
made of Hydrogen and Carbon. Other additives in the petrol also add more elements, but
for the purpose of this article, we'll just look at the hydrocarbons.
Air is a mixture of gasses, mostly Nitrogen which is quite unreactive under normal
conditions, and there is also about 21% Oxygen. The Oxygen reacts with the hydrocarbons when burnt
to produce Carbon Monoxide (CO), Carbon Dioxide (CO2) and Water (H2O).
Under the temperatures and pressures involved, Nitrogen also forms some oxides, collectively
referred to as NOX.
Depending on the temperatures, and ratios of air to fuel, different products will be made,
and also there will be different power outputs for each mix.
- Petrol 14.7:1
- Diesel 14.6:1
- Methanol 6.4:1
- Ethanol 9:1
- LPG 15.5:1
Those are the Air to Fuel ratios for the most common fuels, by mass, known as the Stoichiometric Ratio. The Ratios do not tell the full picture though. They are the perfect ratios for a well mixed air and fuel vapour. In an engine, the mixing takes place very quickly and a perfect mix is impossible. Introductions like Swirl flaps or Disturbed Air Admittance valves agitate the air going in to mix the air and fuel better, but still, a perfect mix can't be attained.
Inside the cylinder will be a cloud of vapour, with some patches richer in fuel, and others leaner. The fuel can be accurately
controlled, so the limiting factor in a Normally Aspirated petrol engine is the amount of air.
To achieve the best power, all of the air has to be used as efficiently as possible, so the mixture will
be enrichened by adding more fuel. The AFR for maximum power is usually between 12-13:1, depending on how well
mixed the vapour was beforehand.
The extra fuel increases the likelihood of the free oxygen being closer to a fuel molecule for the reaction.
Some fuel will be wasted as there is an excess in the cylinder, but it helps ensure all of the air is used to it's full effect.
Likewise, running lean is better for economy. Where economy is concerned, you are looking to use all of the fuel injected by having an excess of air.
Free oxygen taken into the cylinder will be wasted but the priority for an economic tune is
to avoid wasting fuel.
Lean running will increase the temperatures of the combustion, and will also make more of the Nitrogen in the air
oxidise into Nitrogen Oxides (NO and NO2, Nitrous Oxide is N20 and a different kettle of fish).
The excess heat if unchecked can cause engine and Catalytic Converter damage, and the NOX is
an undesirable air pollutant.
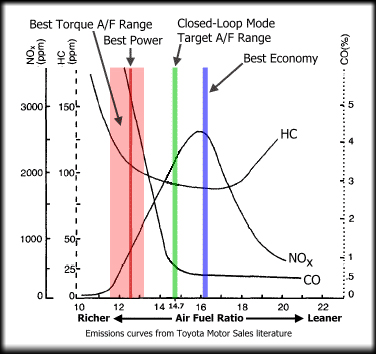 This chart shows the resultant gasses from
burning petrol at different AFRs. Rich mixtures are cooler but you can see the increased
Hydrocarbon emissions as the excess fuel is unused. Nitrogen oxides are low from the cooler temps, but
Carbon Monoxide is far higher with the lack of free oxygen to convert the CO to CO2.
This chart shows the resultant gasses from
burning petrol at different AFRs. Rich mixtures are cooler but you can see the increased
Hydrocarbon emissions as the excess fuel is unused. Nitrogen oxides are low from the cooler temps, but
Carbon Monoxide is far higher with the lack of free oxygen to convert the CO to CO2.
Lean mixtures around 16:1 AFR produce the best economy, but the extra heat oxidises the Nitrogen in the air
increasing air pollution, but with low CO levels. Leaning the mixture further past this point creates lean misfires
with the mixture failing to completely combust, lowering temperatures (and therefore NOx levels).
The Hydrocarbon (HC) levels start to rise as unburnt fuel exits the exhaust and power drops off.
The Stoichiometric ratio of 14.7:1 can be seen on the chart which provides a good compromise between power,
economy and emissions, and this is the target for closed loop operation which will be covered in the next section.
 The Stoichiometric ratio mentioned for different fuels above has the simple name Lambda
and is denoted by the Greek symbol λ.
Figures lower than λ=1 are rich, and higher lambdas are lean.
The Stoichiometric ratio mentioned for different fuels above has the simple name Lambda
and is denoted by the Greek symbol λ.
Figures lower than λ=1 are rich, and higher lambdas are lean.
 The final piece of kit in the exhaust system is the Catalytic Converter. This forces a reaction onto the
remaining exhaust gasses to reduce the pollution. The majority of Cats used today are Three Way Catalytic Converters.
The final piece of kit in the exhaust system is the Catalytic Converter. This forces a reaction onto the
remaining exhaust gasses to reduce the pollution. The majority of Cats used today are Three Way Catalytic Converters.